Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
- Main article: Psychological aspects of Human genetic engineering
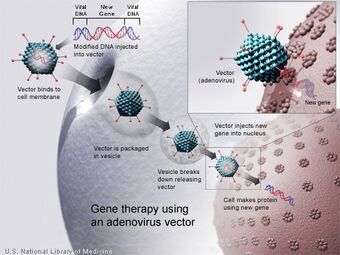
Gene therapy using an Adenovirus vector. A new gene is inserted into an adenovirus vector, which is used to introduce the modified DNA into a human cell. If the treatment is successful, the new gene will make a functional protein.
Gene therapy is the insertion of genes into an individual's cells and tissues to treat a disease, and hereditary diseases in particular. Gene therapy typically aims to supplement a defective mutant allele with a functional one. Although the technology is still in its infancy, it has been used with some success. Antisense therapy is not strictly a form of gene therapy, but is often lumped together with them.
Background[]
In the 1980s, advances in molecular biology had already enabled human genes to be sequenced and cloned. Scientists looking for a method of easily producing proteins — such as insulin, the protein deficient in diabetes mellitus type 1 — investigated introducing human genes to bacterial DNA. The modified bacteria then produce the corresponding protein, which can be harvested and injected in people who cannot produce it naturally.
On September 14, 1990 researchers at the U.S. National Institutes of Health performed the first approved gene therapy procedure on four-year old Ashanti DeSilva. Born with a rare genetic disease called severe combined immunodeficiency (SCID), she lacked a healthy immune system, and was vulnerable to every passing germ. Children with this illness usually develop overwhelming infections and rarely survive to adulthood; a common childhood illness like chickenpox is life-threatening. Ashanti led a cloistered existence--avoiding contact with people outside her family, remaining in the sterile environment of her home, and battling frequent illnesses with massive amounts of antibiotics.
In Ashanti's gene therapy procedure, doctors removed white blood cells from the child's body, let the cells grow in the lab, inserted the missing gene into the cells, and then infused the genetically modified blood cells back into the patient's bloodstream. Laboratory tests have shown that the therapy strengthened Ashanti's immune system; she no longer has recurrent colds, she has been allowed to attend school, and she was immunized against whooping cough. This procedure was not a cure; the white blood cells treated genetically only work for a few months, and the process must be repeated every few months. (VII, Thompson [First] 1993).
Although this simplified explanation of a gene therapy procedure sounds like a happy ending, it is little more than an optimistic first chapter in a long story; the road to the first approved gene therapy procedure was rocky and fraught with controversy. The biology of human gene therapy is very complex, and there are many techniques that still need to be developed and diseases that need to be understood more fully before gene therapy can be used appropriately. The public policy debate surrounding the possible use of genetically engineered material in human subjects has been equally complex. Major participants in the debate have come from the fields of biology, government, law, medicine, philosophy, politics, and religion, each bringing different views to the discussion.
Scientists took the logical step of trying to introduce genes straight into human cells, focusing on diseases caused by single-gene defects, such as cystic fibrosis, hemophilia, muscular dystrophy and sickle cell anemia. However, this has been much harder than modifying simple bacteria, primarily because of the problems involved in carrying large sections of DNA and delivering it to the right site on the genome.
Basic process[]
In most gene therapy studies, a "normal" gene is inserted into the genome to replace an "abnormal," disease-causing gene. A carrier molecule called a vector must be used to deliver the therapeutic gene to the patient's target cells. Currently, the most common vector is a virus that has been genetically altered to carry normal human DNA. Viruses have evolved a way of encapsulating and delivering their genes to human cells in a pathogenic manner. Scientists have tried to take advantage of this capability and manipulate the virus genome to remove disease-causing genes and insert therapeutic genes.
Target cells such as the patient's liver or lung cells are infected with the vector. The vector then unloads its genetic material containing the therapeutic human gene into the target cell. The generation of a functional protein product from the therapeutic gene restores the target cell to a normal state.
Types of gene therapy[]
In theory it is possible to transform either somatic cells (most cells of the body) or cells of the germline (such as sperm cells, ova, and their stem cell precursors). All gene therapy so far in people has been directed at somatic cells, whereas germline engineering in humans remains only a highly controversial prospect. For the introduced gene to be transmitted normally to offspring, it needs not only to be inserted into the cell, but also to be incorporated into the chromosomes by genetic recombination.
Somatic gene therapy can be broadly split in to two categories: ex vivo (where cells are modified outside the body and then transplanted back in again) and in vivo (where genes are changed in cells still in the body.) Recombination-based approaches in vivo are especially uncommon, because for most DNA constructs recombination has a very low probability.
Broad methods[]
There are a variety of different methods to replace or repair the genes targeted in gene therapy.
- A normal gene may be inserted into a nonspecific location within the genome to replace a nonfunctional gene. This approach is most common.[How to reference and link to summary or text]
- An abnormal gene could be swapped for a normal gene through homologous recombination.
- The abnormal gene could be repaired through selective reverse mutation, which returns the gene to its normal function.
- The regulation (the degree to which a gene is turned on or off) of a particular gene could be altered.
Vectors in gene therapy[]
Viruses[]
- Main article: Viral vector
All viruses attack their hosts and introduce their genetic material into the host cell as part of their replication cycle. This genetic material contains basic 'instructions' of how to produce more copies of these viruses, hijacking the body's normal production machinery to serve the needs of the virus. The host cell will carry out these instructions and produce additional copies of virus, leading to more and more cells becoming infected. Some types of viruses actually physically insert their genes into the host's genome (it is the defining feature of retroviruses, the family of viruses that includes HIV, the virus that causes AIDS). This incorporates the genes of that virus among the genes of the host cell for the life span of that cell.
Doctors and molecular biologists realized that viruses like this could be used as vehicles to carry 'good' genes into a human cell. First, a scientist would remove the genes in the virus that cause disease. Then they would replace those genes with genes encoding the desired effect (for instance, insulin production in the case of diabetics). This procedure must be done in such a way that the genes which allow the virus to insert its genome into its host's genome are left intact. This can be confusing, and requires significant research and understanding of the virus' genes in order to know the function of each. An example:
A virus is found which replicates by inserting its genes into the host cell's genome. This virus has two genes- A and B. Gene A encodes a protein which allows this virus to insert itself into the host's genome. Gene B actually causes the disease this virus is associated with. Gene C is the "normal" or "desirable" gene we want in the place of gene B. Thus, by re-engineering the virus so that gene B is replaced by gene C, while allowing gene A to properly function, this virus could introduce your 'good gene'- gene C into the host cell's genome without causing any disease.
All this is clearly an oversimplification, and numerous problems exist that prevent gene therapy using viral vectors, such as: trouble preventing undesired effects, ensuring the virus will infect the correct target cell in the body, and ensuring that the inserted gene doesn't disrupt any vital genes already in the genome. However, this basic mode of gene introduction currently shows much promise and doctors and scientists are working hard to fix any potential problems that could exist.
Retroviruses[]
The genetic material in retroviruses is in the form of RNA molecules, while the genetic material of their hosts is in the form of DNA. When a retrovirus infects a host cell, it will introduce its RNA together with some enzymes into the cell. This RNA molecule from the retrovirus must produce a DNA copy from its RNA molecule before it can be considered for part of the genetic material of the host cell. The process of producing a DNA copy from an RNA molecule is termed reverse transcription. It is carried out by one of the enzymes carried in the virus, called reverse transcriptase. After this DNA copy is produced and is free in the nucleus of the host cell, it must be incorporated into the genome of the host cell. That is, it must be inserted into the large DNA molecules in the cell (the chromosomes). This process is done by another enzyme carried in the virus called integrase.
Now that the genetic material of the virus is incorporated and has become part of the genetic material of the host cell, we can say that the host cell is now modified to contain a new gene. If this host cell divides later, its descendants will all contain the new genes.
One of the problems of gene therapy using retroviruses is that the integrase enzyme can insert the genetic material of the virus in any arbitrary position in the genome of the host. If genetic material happens to be inserted in the middle of one of the original genes of the host cell, this gene will be disrupted (insertional mutagenesis). If the gene happens to be one regulating cell division, uncontrolled cell division (i.e., cancer) can occur. This problem has recently begun to be addressed by utilizing zinc finger nucleases[1] or by including certain sequences such as the beta-globin locus control region[6] to direct the site of integration to specific chromosomal sites.
Gene therapy trials to treat severe combined immunodeficiency (SCID) were halted or restricted in the USA when leukemia was reported in three of eleven patients treated in the French Therapy X-linked SCID (XSCID) gene therapy trial. Ten XSCID patients treated in England have not presented leukemia to date and have had similar success in immune reconstitution. Gene therapy trials to treat SCID due to deficiency of the Adenosine Deaminase (ADA) enzyme continue with relative success in the USA, Italy and Japan.
Adenoviruses[]
Adenoviruses are viruses that carry their genetic material in the form of double-stranded DNA. They cause respiratory (especially the common cold), intestinal, and eye infections in humans. When these viruses infect a host cell, they introduce their DNA molecule into the host. The genetic material of the adenoviruses is not incorporated into the host cell's genetic material. The DNA molecule is left free in the nucleus of the host cell, and the instructions in this extra DNA molecule are transcribed just like any other gene. The only difference is that these extra genes are not replicated when the cell is about to undergo cell division so the descendants of that cell will not have the extra gene. As a result, treatment with the adenovirus will require readministration in a growing cell population although the absence of integration into the host cell's genome should prevent the type of cancer seen in the SCID trials. This vector system has shown real promise in treating cancer and indeed the first gene therapy product to be licenced is an adenovirus to treat cancer.
Adeno-associated viruses[]
Adeno-associated viruses, from the parvovirus family, are small viruses with a genome of single stranded DNA. These viruses can insert genetic material at a specific site on chromosome 19. There are a few disadvantages to using AAV, including the small amount of DNA it can carry (low capacity) and the difficulty in producing it. This type of virus is being used, however, because it is non-pathogenic (most people carry this harmless virus). In contrast to adenoviruses, most people treated with AAV will not build an immune response to remove the virus and the cells that have been successfully treated with it. Several trials with AAV are on-going or in preparation, mainly trying to treat muscle and eye diseases; the two tissues where the virus seems particularly useful. However, clinical trials have also been initiated where AAV vectors are used to deliver genes to the brain. This is possible because AAV viruses can infect non-dividing (quiescent) cells, such as neurons in which their genomes be expressed for a long time. In recent human trials, CD8+ immune cells have recognised the AAV infected cells as compromised and killed these cells accordingly. This action appears to be triggered by part of the capsid or outer coat of the type 2 virus. Recent studies have shown that humans will likely react in the same way against the new serotype 8 AAV as well.
Envelope protein pseudotyping of viral vectors[]
The viral vectors described above have natural host cell populations that they infect most efficiently. Retroviruses have limited natural host cell ranges, and although adenovirus and adeno-associated virus are able to infect a relatively broader range of cells efficiently, some cell types are refractory to infection by these viruses as well. Attachment to and entry into a susceptible cell is mediated by the protein envelope on the surface of a virus. Retroviruses and adeno-associated viruses have a single protein coating their membrane, while adenoviruses are coated with both an envelope protein and fibers that extend away from the surface of the virus. The envelope proteins on each of these viruses bind to cell-surface molecules such as heparin sulfate, which localizes them upon the surface of the potential host, as well as with the specific protein receptor that either induces entry-promoting structural changes in the viral protein, or localizes the virus in endosomes wherein acidification of the lumen induces this refolding of the viral coat. In either case, entry into potential host cells requires a favorable interaction between a protein on the surface of the virus and a protein on the surface of the cell. For the purposes of gene therapy, one might either want to limit or expand the range of cells susceptible to transduction by a gene therapy vector. To this end, many vectors have been developed in which the endogenous viral envelope proteins have been replaced by either envelope proteins from other viruses, or by chimeric proteins. Such chimera would consist of those parts of the viral protein necessary for incorporation into the virion as well as sequences meant to interact with specific host cell proteins. Viruses in which the envelope proteins have been replaced as described are referred to as pseudotyped viruses. For example, the most popular retroviral vector for use in gene therapy trials has been the lentivirus Simian Immunodeficiency virus coated with the envelope proteins, G-protein, from Vesicular Stomatitus virus. This vector is referred to as VSV G-pseudotyped lentivirus, and infects an almost universal set of cells. This tropism is characteristic of the VSV G-protein with which this vector is coated. Many attempts have been made to limit the tropism of viral vectors to one or a few host cell populations. This advance would allow for the systemic administration of a relatively small amount of vector. The potential for off-target cell modification would be limited, as well as many concerns from the medical community. Most attempts to limit tropism have used chimeric envelope proteins bearing antibody fragments. These vectors show great promise for the development of "magic bullet" gene therapies.
Non-viral methods[]
Non-viral methods present certain advantages over viral methods; simple large scale production and low host immunogenicity being just two. Previously, low levels of transfection and expression of the gene held non-viral methods at a disadvantage, however recent advances in vector technology has yielded molecules and techniques with transfection efficiencies similar to that of viruses.
Naked DNA[]
This is the simplest method of non-viral transfection. Clinical trials have been carried out of intramuscular injection of a naked DNA plasmid have occurred with some success, however the expression has been very low in comparison to other methods of transfection. In addition to trials with plasmids, there have been trials with naked PCR product, which have had similar or greater success, however this success does not compare to that of the other methods, leading to research into more efficient methods for delivery of the naked DNA such as electroporation and the use of a "gene gun", which shoots DNA coated gold particles into the cell using high pressure gas.
Oligodeoxynucleotides[]
The use of synthetic oligodeoxynucleotides in gene therapy is to inactivate the genes involved in the disease process. There are several methods by which this is achieved. One strategy uses antisense specific to the target gene to disrupt the transcription of the faulty gene. Another uses small catalytic molecules of RNA called siRNA to cleave specific unique sequences in the mRNA transcript of the faulty gene, disrupting translation of the faulty mRNA, and therefore expression of the gene. A further strategy uses double stranded oligodeoxynucleotides as a decoy for the transcription factors that are required to activate the transcription of the target gene. The transcription factors bind to the decoys instead of the promoter of the faulty gene which reduces the transcription of the target gene, lowering expression. pp
Lipoplexes and polyplexes[]
To improve the delivery of the new DNA into the cell, the DNA must be protected from damage and its entry into the cell must be facilitated. To this end new molecules, lipoplexes and polyplexes, have been created that have the ability to protect the DNA from undesirable degradation during the transfection process.
Plasmid DNA can be covered with lipids in an organized structure like a micelle or a liposome. When the organized structure is complexed with DNA it is called a lipoplex. There are three types of lipoplexes, anionic (negatively charged), neutral or cationic (positively charged). Initially, anionic and neutral lipids were used for the construction of lipoplexes for synthetic vectors. However, although there is little toxicity associated with them, they are compatible with body fluids and there was a possibility of adapting them to be tissue specific, they are complicated and time consuming to produce so attention was turned to the cationic versions.
Cationic lipids, due to their positive charge, naturally complex with the negatively charged DNA. Also as a result of their charge they interact with the cell membrane, endocytosis of the lipoplex occurs and the DNA is released into the cytoplasm. The cationic lipids also protect against degradation of the DNA by the cell.
The most common use of lipoplexes has been in gene transfer into cancer cells, where the supplied genes have activated tumor suppressor control genes in the cell and decrease the activity of oncogenes. Recent studies have shown lipoplexes to be useful in transfecting respiratory epithelial cells, so they may be used for treatment of genetic respiratory diseases such as cystic fibrosis.
Complexes of polymers with DNA are called polyplexes. Most polyplexes consist of cationic polymers and their production is regulated by ionic interactions. One large difference between the methods of action of polyplexes and lipoplxes is that polyplexes cannot release their DNA load into the cytoplasm, so to this end, co-transfection with endosome-lytic agents (to lyse the endosome that is made during endocytosis, the process by which the polyplex enters the cell) such as inactivated adenovirus must occur. However this isn't always the case, polymers such as polyethylenimine have their own method of endosome disruption.
Hybrid methods[]
Due to every method of gene transfer having shortcomings, there has been some hybrid methods developed that combine two or more techniques. Virosomes are one example; they combine liposomes with an inactivated HIV or influenza virus. This has been shown to have more efficient gene transfer in respiratory epithelial cells than either viral or liposomal methods alone. Other methods involve mixing other viral vectors with cationic lipids or hybridising viruses.
Recent developments in gene therapy[]
Scientist at the National Institutes of Health (Bethesda, MD) have successfully treated metastatic melanoma in two patients using killer T cells genetically retargeted to attack the cancer cells. This study constitutes the first demonstration that gene therapy can be effective in treating cancer. The study results have been submitted for publication.
In May 2006 a team of scientists led by Drs. Luigi Naldini and Brian Brown from the San Raffaele Telethon Institute for Gene Therapy (HSR-TIGET) in Milan, Italy reported a breakthrough for gene therapy in which they developed a way to prevent the immune system from rejecting a newly delivered gene. Similar to organ transplanation, gene therapy has been plagued by the problem of immune rejection. So far, delivery of the 'good' gene has been difficult because the immune sytem does not recognize the new gene and rejects the cells carrying it. To overcome this problem, the HSR-TIGET group utilized a newly uncovered network of genes regulated by molecules known as microRNAs. Dr. Naldini's group reasoned that they could use this natural function of microRNA to selectively turn off the identity of their therapeutic gene in cells of the immune system and prevent the gene from being found and destroyed. The researchers injected mice with the gene containing an immune-cell microRNA target sequence, and spectacularly, the mice did not reject the gene, as previously occurred when vectors without the microRNA target sequence were used. This work will have important implications for the treatment of hemophilia and other genetic diseases by gene therapy [1].
In March 2006 an international group of scientists announced the successful use of gene therapy to treat two adult patients for a disease affecting myeloid cells. The study, published in Nature Medicine, is believed to be the first to show that gene therapy can cure diseases of the myeloid system [2]
University of California, Los Angeles, research team gets genes into the brain using liposomes coated in a polymer called polyethylene glycol (PEG). The transfer of genes into the brain is a significant achievement because viral vectors are too big to get across the "blood-brain barrier." This method has potential for treating Parkinson's disease. See Undercover genes slip into the brain at NewScientist.com (March 20, 2003).
RNA interference or gene silencing may be a new way to treat Huntington's. Short pieces of double-stranded RNA (short, interfering RNAs or siRNAs) are used by cells to degrade RNA of a particular sequence. If a siRNA is designed to match the RNA copied from a faulty gene, then the abnormal protein product of that gene will not be produced. See Gene therapy may switch off Huntington's at NewScientist.com (March 13, 2003).
New gene therapy approach repairs errors in messenger RNA derived from defective genes. Technique has potential to treat the blood disorder thalassaemia, cystic fibrosis, and some cancers. See Subtle gene therapy tackles blood disorder at NewScientist.com (October 11, 2002).
Researchers at Case Western Reserve University and Copernicus Therapeutics are able to create tiny liposomes 25 nanometers across that can carry therapeutic DNA through pores in the nuclear membrane. See DNA nanoballs boost gene therapy at NewScientist.com (May 12, 2002).
Sickle cell is successfully treated in mice. See Murine Gene Therapy Corrects Symptoms of Sickle Cell Disease from March 18, 2002, issue of The Scientist.
The success of a multi-center trial for treating children with SCID (severe combined immune deficiency or "bubble boy" disease) held from 2000 and 2002 was questioned when two of the ten children treated at the trial's Paris center developed a leukemia-like condition. Clinical trials were halted temporarily in 2002, but resumed after regulatory review of the protocol in the United States, the United Kingdom, France, Italy, and Germany. (V. Cavazzana-Calvo, Thrasher and Mavilio 2004; see also 'Miracle' gene therapy trial halted at NewScientist.com, October 3, 2002).
Problems and ethics[]
For the safety of gene therapy, the Weismann barrier is fundamental in the current thinking. Soma-to-germline feedback should therefore be impossible. However, there are indications [3] that the Weissman barrier can be breached. One way it might possibly be breached is if the treatment were somehow misapplied and spread to the testes and therefore would infect the germline against the intentions of the therapy.
Some of the problems of Gene Therapy include:
• Short-lived nature of gene therapy - Before gene therapy can become a permanent cure for any condition, the therapeutic DNA introduced into target cells must remain functional and the cells containing the therapeutic DNA must be long-lived and stable. Problems with integrating therapeutic DNA into the genome and the rapidly dividing nature of many cells prevent gene therapy from achieving any long-term benefits. Patients will have to undergo multiple rounds of gene therapy.
• Immune response - Anytime a foreign object is introduced into human tissues, the immune system is designed to attack the invader. The risk of stimulating the immune system in a way that reduces gene therapy effectiveness is always a potential risk. Furthermore, the immune system's enhanced response to invaders it has seen before makes it difficult for gene therapy to be repeated in patients.
• Problems with viral vectors - Viruses, while the carrier of choice in most gene therapy studies, present a variety of potential problems to the patient --toxicity, immune and inflammatory responses, and gene control and targeting issues. In addition, there is always the fear that the viral vector, once inside the patient, may recover its ability to cause disease.
• Multigene disorders - Conditions or disorders that arise from mutations in a single gene are the best candidates for gene therapy. Unfortunately, some of the most commonly occurring disorders, such as heart disease, high blood pressure, Alzheimer's disease, arthritis, and diabetes, are caused by the combined effects of variations in many genes. Multigene or multifactorial disorders such as these would be especially difficult to treat effectively using gene therapy.
• Chance of inducing a tumor - If the DNA is integrated in the wrong place in the genome, for example in a tumor suppressor gene, it could induce a tumor.
In popular culture[]
Gene therapy plays a major role in the sci-fi series Stargate Atlantis, as a certain type of alien technology can only be used if one has a certain gene which is given to the members of the team through gene therapy. Gene therapy also plays a major role in the plot of the James Bond movie Die Another Day. The Yellow Bastard from Frank Miller's Sin City was also apparently the receipent of gene therapy. Gene therapy is a crucial plot element in the video game Metal Gear Solid, where it has been used to enhance the battle capabilities of enemy soldiers.
Publications[]
Molecular Therapy is the official journal of the American Society of Gene Therapy, and is a rapid-publication, peer-reviewed journal covering all aspects of human gene, cell, protein and nucleic acid therapy.
Human Gene Therapy, published by Mary Ann Liebert, Inc., is a rapid-publication, peer-reviewed journal covering all aspects of human gene therapy.
See also[]
- Antisense therapy
- DNA
- Genetic engineering
- Life extension
- List of life extension related topics
- Pharmacological Gene Therapy
- Technology assessment
External links[]
- Gene Therapy: Molecular Bandage? University of Utah's Genetic Science Learning Center
- The American Society of Gene Therapy
- The European Society of Gene Therapy
- 2003 news relating to gene therapy
- Research Group at Cambridge, UK working on an overcoming current hurdles to successful gene therapy
- Council for Responsible Genetics
References[]
- Durai, Sundar, Mala Mani, Karthikeyan Kandavelou, Joy Wu, Matthew H. Porteus, and Srinivasan Chandrasegaran (October 2005). Zinc finger nucleases: custom-designed molecular scissors for genome engineering of plant and mammalian cells. Nucleic Acids Research 33 (18): 5978–5990. PMID 16251401.
- Gardlik, Roman, Roland Pálffy, Július Hodosy, Ján Lukács, Ján Turňa and Peter Celec (April 2005). Vectors and delivery systems in gene therapy. Medical Science Monitor 11 (4): 110–121. PMID 15795707.
- Staff Gene Therapy. (FAQ) Human Genome Project Information. Oak Ridge National Laboratory. URL accessed on May 28, 2006.
- Baum C, Dullmann J, Li Z, Fehse B, Meyer J, Williams DA, von Kalle C. Side effects of retroviral gene transfer into hematopoietic stem cells. Blood. 2003 Mar 15;101(6):2099-114
- Horn PA, Morris JC, Neff T, Kiem HP. Stem cell gene transfer--efficacy and safety in large animal studies. Molecular Therapy, 2004 Sep;10(3):417-31
- Wang, Hongjie, Dmitry M. Shayakhmetov, Tobias Leege, Michael Harkey, Qiliang Li, Thalia Papayannopoulou, George Stamatoyannopolous, and André Lieber (September 2005). A capsid-modified helper-dependent adenovirus vector containing the beta-globin locus control region displays a nonrandom integration pattern and allows stable, erythroid-specific gene expression. Journal of Virology 79 (17): 10999-11013.
Further reading[]
- Hall, Steven S. (1997) A Commotion in the Blood. New York, New York: Henry Holt and Company. ISBN 0-8050-5841-9
ca:Teràpia gènica da:Genterapi de:Gentherapie es:Terapia génica eo:Genterapio fr:Thérapie génique he:ריפוי גני nl:Gentherapie no:Genterapi pt:Terapia genética ru:Генотерапия fi:Geeniterapia sv:Genterapi ur:وراثی معالجہ zh:基因治療
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |