Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
| Indole | |
|---|---|

| |
| General | |
| Systematic name | Indole |
| Other names | 2,3-Benzopyrrole, ketole, 1-benzazole |
| Molecular formula | C8H7N |
| SMILES | C1(NC=C2)=C2C=CC=C1 |
| Molar mass | 117.15 g/mol |
| Appearance | White solid |
| CAS number | 120-72-9 |
| Properties | |
| Density and phase | 1.22 g/cm3, solid |
| Solubility in water | 0.19 g/100 ml (20 °C) Soluble in hot water |
| In ethanol, ether In benzene |
Highly soluble Soluble |
| Melting point | 52 - 54°C (326 K) |
| Boiling point | 253 - 254°C (526 K) |
| Acidity (pKa) | 16.2 (21.0 in DMSO) |
| Basicity (pKb) | 17.6 |
| Structure | |
| Molecular shape | Planar |
| Crystal structure | ? |
| Dipole moment | 2.11 D in benzene |
| Hazards | |
| MSDS | External MSDS |
| Main hazards | ? |
| NFPA 704 | |
| Flash point | 121°C |
| R/S statement | R: 21/22-37/38-41-50/53 S: 26-36/37/39-60-61 |
| RTECS number | NL2450000 |
| Supplementary data page | |
| Structure and properties |
n, εr, etc. |
| Thermodynamic data |
Phase behaviour Solid, liquid, gas |
| Spectral data | UV, IR, NMR, MS |
| Related compounds | |
| Related aromatic compounds |
benzene, benzofuran, carbazole, carboline, indene, indoline, isatin, methylindole, oxindole, pyrrole, skatole |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references | |
Indole is an aromatic heterocyclic organic compound. It has a bicyclic structure, consisting of a six-membered benzene ring fused to a five-membered nitrogen-containing pyrrole ring. The participation of the nitrogen lone electron pair in the aromatic ring means that indole is not a base, and it does not behave like a simple amine.
Indole is solid at room temperature. It occurs naturally in human feces and has an intense fecal smell. At very low concentrations, however, it has a flowery smell, and is a constituent of many flower scents (such as orange blossoms) and perfumes. It also occurs in coal tar.
The indole structure can be found in many organic compounds like the amino acid tryptophan and in tryptophan-containing protein, in alkaloids, and in pigments.
Indole undergoes electrophilic substitution, mainly at position 3. Substituted indoles are structural elements of (and for some compounds the synthetic precursors for) the tryptophan-derived tryptamine alkaloids like the neurotransmitter serotonin, melatonin, the hallucinogens psilocybin, DMT, 5-MeO-DMT, or the ergolines like LSD. Other indolic compounds include the plant hormone Auxin (indolyl-3-acetic acid, IAA), the anti-inflammatory drug indomethacin, and the betablocker pindolol.
The name indole is derived from a combination of the words indigo and oleum, since indole was first isolated by treatment of the indigo dye with oleum.
History[]
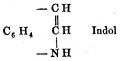
Baeyer's original structure for indole, 1869
Indole chemistry began to develop with the study of the dye indigo. This was converted to isatin and then to oxindole. Then, in 1866, Adolf von Baeyer reduced oxindole to indole using zinc dust.[1] In 1869, he proposed the formula for indole (left) that is accepted today.[2]
Certain indole derivatives were important dyestuffs until the end of the 19th century. In the 1930s, interest in indole intensified when it became known that the indole nucleus is present in many important alkaloids, as well is in tryptophan and auxins, and it remains an active area of research today.[3]
Synthesis of indoles[]
Indole is a major constituent of coal-tar, and the 220-260 °C distillation fraction is the main industrial source of the material. Indole and its derivatives can also be synthesized by a variety of methods.[4][5][6]
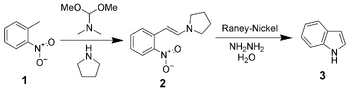
Leimgruber-Batcho indole synthesis[]
The Leimgruber-Batcho indole synthesis is an efficient method of sythesizing indole and substituted indoles. Originally disclosed in a patent in 1976, this method is high-yielding and can generate substituted indoles. This method is especially popular in the pharmaceutical industry, where many pharmaceutical drugs are comprised of specifically substituted indoles.
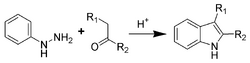
Fischer indole synthesis[]
One of the oldest and most reliable methods for synthesizing substituted indoles is the Fischer indole synthesis developed in 1883 by Emil Fischer. Although the synthesis of indole itself is problematic using the Fischer indole synthesis, it is often used to generate indoles substituted in the 2- and/or 3-positions.
Other indole forming reactions[]
- Bartoli indole synthesis
- Bischler-Möhlau indole synthesis
- Gassman indole synthesis
- Hemetsberger indole synthesis
- Larock indole synthesis
- Madelung synthesis
- Nenitzescu indole synthesis
- Reissert indole synthesis
Chemical reactions of indole[]
Nitrogen basicity[]
Although the indole N-1 nitrogen atom has a lone pair of electrons, indole is not basic like amines and anilines because the lone pair is delocalised and contributes to the aromatic system. The protonated form has an pKa of -3.6, so that very strong acids like hydrochloric acid are needed to protonate a substantial amount of indole. The sensitivity of many indolic compounds (e.g., tryptamines) under acidic conditions is caused by this protonation.
Electrophilic substitution[]
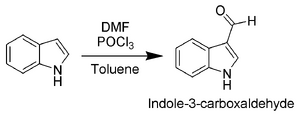
The most reactive position on indole for electrophilic aromatic substitution is C-3, which is 1013 times more reactive than benzene. For example, Vilsmeier-Haack formylation of indole[7] will take place at room temperature exclusively at C-3. Since the pyrrollic ring is the most reactive portion of indole, nucleophilic substitution of the carbocyclic (benzene) ring can take place only after N-1, C-2, and C-3 are substituted.
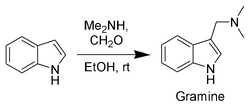
Gramine, a useful synthetic intermediate, is produced via a Mannich reaction of indole with dimethylamine and formaldehyde.
Nitrogen-H acidity and organometallic indole anion complexes[]
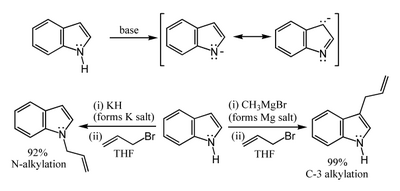
The N-H proton has a pKa of 21 in DMSO, so that very strong bases like sodium hydride or butyl lithium and water-free conditions are needed for complete deprotonation. Salts of the resulting indole anion can react in two ways. Highly-ionic salts such as the sodium or potassium compounds tend to react with electrophiles at nitrogen-1, whereas the more covalent magnesium compounds (indole Grignard reagents) and (especially) zinc complexes tend to react at carbon-3 (see figure below). For the same reason, polar aprotic solvents such as DMF and DMSO tend to favour attack at the nitrogen, whereas nonpolar solvents such as toluene favour C-3 attack.[8]
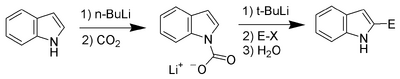
Carbon acidity and C-2 lithiation[]
After the N-H proton, the hydrogen at C-2 is the next most acidic proton on indole. Reaction of N-protected indoles with butyl lithium or lithium diisopropylamide results in lithiation exclusively at the C-2 position. This strong nucleophile can then be used as such with other electrophiles.
Bergman and Venemalm developed a technique for lithiating the 2-position of unsubstituted indole.[9]
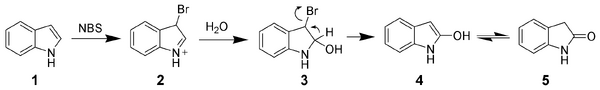
Oxidation of indole[]
Due to the electron-rich nature of indole, it is easily oxidized. Simple oxidants such as N-bromosuccinimide will selectively oxidize indole 1 to oxindole (4 and 5).
Cycloadditions of indole[]
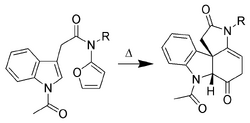
Only the C-2 to C-3 pi-bond of indole is capable of cycloaddition reactions. Intermolecular cycloadditions are not favorable, whereas intramolecular variants are often high-yielding. For example, Padwa et al.[10] have developed this Diels-Alder reaction to form advanced strychnine intermediates. In this case, the 2-aminofuran is the diene, whereas the indole is the dienophile.
Indoles also undergo intramolecular [2+3] and [2+2] cycloadditions.
Applications[]
Natural jasmine oil, used in the perfume industry, contains around 2.5% of indole. Since 1 kg of the natural oil requires processing several million jasmine blossoms and costs around $10,000, it is not surprising that indole (among other things) is used in the manufacture of synthetic jasmine oil (which costs around $10/kg).
See also[]
General references[]
- Indoles Part One, W. J. Houlihan (ed.), Wiley Interscience, New York, 1972.
- Sundberg, R. J. (1996). Indoles, San Diego: Academic Press. ISBN 0-12-676945-1.
- Joule, J. A.; Mills, K. (2000). Heterocyclic Chemistry, Oxford, UK: Blackwell Science. ISBN 0-632-05453-0.
- Joule, J., In Science of Synthesis, Thomas, E. J., Ed.; Thieme: Stuttgart, (2000); Vol. 10, p. 361. ISBN 3-13-112241-2 (GTV); ISBN 0-86577-949-X (TNY).
References[]
- ↑ Baeyer, A. Ann. 1866, 140, 295.
- ↑ Baeyer, A.; Emmerling, A. Chemische Berichte 1869, 2, 679.
- ↑ R. B. Van Order, H. G. Lindwall Chem. Rev. 1942, 30, 69-96. (Review) (
- REDIRECT Template:Doi
- ↑ Gribble G. W. J. Chem. Soc. Perkin Trans. 1 2000, 1045-1075. (Review) (
- REDIRECT Template:Doi
- ↑ Cacchi, S.; Fabrizi, G. Chem. Rev. 2005, 105, 2873-2920. (Review) (
- REDIRECT Template:Doi
- ↑ Humphrey, G. R.; Kuethe, J. T. Chem. Rev. 2006, 106, 2875-2911. (Review) (
- REDIRECT Template:Doi
- ↑ James, P. N.; Snyder, H. R. (1959). Indole-3-aldehyde. Organic Syntheses 39: 30.
- ↑ Heaney, H.; Ley, S. V. (1974). 1-Benzylindole. Organic Syntheses 54: 58.
- ↑ Bergman, J.; Venemalm, L. J. Org. Chem. 1992, 57, 2495 - 2497. (
- REDIRECT Template:Doi
- ↑ Lynch, S. M. ; Bur, S. K.; Padwa, A.; Org. Lett. 2002, 4, 4643 - 4645. (
- REDIRECT Template:Doi
External links[]
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |