| Cytosine | |
|---|---|
| Chemical name | 4-Aminopyrimidin-2(1H)-one |
| Chemical formula | C4H5N3O |
| Molecular mass | 111.10 g/mol |
| Melting point | 320 - 325°C (decomp) |
| CAS number | 71-30-7 |
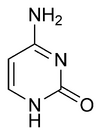
| SMILES | NC1=NC(NC=C1)=O |
| |
Cytosine is one of the 5 main nucleobases used in storing and transporting genetic information within a cell in the nucleic acids DNA and RNA. It is a pyrimidine derivative, with a heterocyclic aromatic ring and two substituents attached (an amine group at position 4 and a keto group at position 2). The nucleoside of cytosine is cytidine. In Watson-Crick base pairing, it forms three hydrogen bonds with guanine.
Cytosine was first discovered in 1894 when it was isolated from calf thymus tissues. A structure was proposed in 1903, and was synthesized (and thus confirmed) in the laboratory in the same year.
Cytosine recently found use in quantum computation. The first time any quantum mechanical properties were harnessed to process information took place on August 1st in 1998 when researchers at Oxford implemented David Deutsch's algorithm on a two qubit NMRQC (Nuclear Magnetic Resonance Quantum Computer) based on the cytosine molecule.
Cytosine can be found as part of DNA, RNA, or as a part of a nucleotide. As cytidine triphosphate (CTP), it can act as a co-factor to enzymes, and can transfer a phosphate to convert adenosine diphosphate (ADP) to adenosine triphosphate (ATP).
In DNA and RNA, cytosine is paired with guanine. However, it is inherently unstable, and can change into uracil (spontaneous deamination). This can lead to a point mutation if not repaired by the DNA repair enzymes.
Cytosine can also be methylated into 5-methylcytosine by an enzyme called DNA methyltransferase.
External links[]
— 4-amino-3H-pyrimidin-2-one
- CID 5274263 from PubChem
— 4-aminopyrimidin-2-ol
- EINECS number 200-749-5
- Computational Chemistry Wiki
- Prebiotic cytosine synthesis: A critical analysis and implications for the origin of life
- Link page to external chemical sources.
| Nucleic acids edit |
|---|
| Nucleobases: Adenine - Thymine - Uracil - Guanine - Cytosine - Purine - Pyrimidine |
| Nucleosides: Adenosine - Uridine - Guanosine - Cytidine - Deoxyadenosine - Thymidine - Deoxyguanosine - Deoxycytidine |
| Nucleotides: AMP - UMP - GMP - CMP - ADP - UDP - GDP - CDP - ATP - UTP - GTP - CTP - cAMP - cGMP |
| Deoxynucleotides: dAMP - dTMP - dUMP - dGMP - dCMP - dADP - dTDP - dUDP - dGDP - dCDP - dATP - dTTP - dUTP - dGTP - dCTP |
| Nucleic acids: DNA - RNA - LNA - PNA - mRNA - ncRNA - miRNA - rRNA - siRNA - tRNA - cDNA - snRNA - snoRNA - mtDNA - Oligonucleotide
|
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |