Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
In chemistry, a catalyst (Greek: καταλύτης, catalytēs) is a substance that decreases the activation energy of a chemical reaction (see also catalysis) without itself being changed at the end of the chemical reaction. Catalysts participate in reactions but are neither reactants nor products of the reaction they catalyse (a strange 'exception' is the process of autocatalysis). They work by providing an alternative pathway for the reaction to occur, thus reducing the activation energy and increasing the reaction rate. More generally, one may at times call anything that accelerates a reaction, without itself being consumed or changed, a "catalyst" (for example, a "catalyst for political change").
A promoter is an accelerator of catalysis, but not a catalyst by itself. An inhibitor inhibits the working of a catalyst.
Definitions[]
Catalysts generally react with one or more reactants to form a chemical intermediate that subsequently reacts to form the final reaction product, in the process regenerating the catalyst. The following is a typical reaction scheme, where C represents the catalyst, A and B are reactants, D is the product of the reaction of A and B:
- A + C → AC (1)
- B + AC → ABC (2)
- ABC → CD (3)
- CD → C + D (4)
Although the catalyst (C) is consumed by reaction 1, it is subsequently produced by reaction 4, so for the overall reaction:
- A + B + C → D + C
the catalyst is neither consumed nor produced.
Catalysts and reaction energetics[]
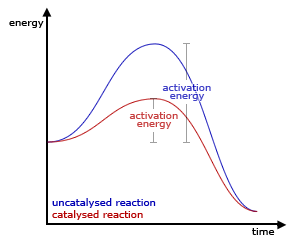
Generic graph showing the effect of a catalyst in an hypothetical exothermic chemical reaction. Notice that the catalysed (red) pathway, despite having a lower activation energy, produces the same final result.
Catalysts work by providing an (alternative) mechanism involving a different transition state and lower activation energy. The effect of this is that more molecular collisions have the energy needed to reach the transition state. Hence, catalysts can perform reactions that, albeit thermodynamically feasible, would not run without the presence of a catalyst, or perform them much faster, more specific, or at lower temperatures. This can be observed on a Boltzmann distribution and energy profile diagram. This means that catalysts reduce the amount of energy needed to start a chemical reaction.
Catalysts cannot make energetically unfavorable reactions possible — they have no effect on the chemical equilibrium of a reaction because the rate of both the forward and the reverse reaction are equally affected (see also thermodynamics). The net free energy change of a reaction is the same whether a catalyst is used or not; the catalyst just makes it easier to activate.
The SI derived unit for measuring the catalytic activity of a catalyst is the katal, which is moles per second. The degree of activity of a catalyst can also be described by the turn over number or TON and the catalytic efficiency by the turn over frequency (TOF). The biochemical equivalent is the enzyme unit.
Types of catalysts[]
Catalysts can be either heterogeneous or homogeneous. Biocatalysis is often seen as a separate group.
Heterogeneous catalysts are present in different phases from the reactants (for example, a solid catalyst in a liquid reaction mixture), whereas homogeneous catalysts are in the same phase (for example, a dissolved catalyst in a liquid reaction mixture).
Heterogeneous catalysts[]
A simple model for heterogeneous catalysis involves the catalyst providing a surface on which the reactants (or substrates) temporarily become adsorbed. Bonds in the substrate become weakened sufficiently for new bonds to be created. The bonds between the products and the catalyst are weaker, so the products are released. Different possible mechanisms for reactions on surfaces are known, depending on how the adsorption takes place (Langmuir-Hinshelwood and Eley-Rideal).
For example, in the Haber process to manufacture ammonia, finely divided iron acts as a heterogeneous catalyst. Active sites on the metal allow partial weak bonding to the reactant gases, which are adsorbed onto the metal surface. As a result, the bond within the molecule of a reactant is weakened and the reactant molecules are held in close proximity to each other. In this way the particularly strong triple bond in nitrogen is weakened and the hydrogen and nitrogen molecules are brought closer together than would be the case in the gas phase, so the rate of reaction increases.
Other heterogeneous catalysts include vanadium(V) oxide in the Contact process, nickel in the manufacture of margarine, alumina and silica in the cracking of alkanes and platinum rhodium palladium in catalytic converters.
In car engines, incomplete combustion of the fuel produces carbon monoxide, which is toxic. The electric spark and high temperatures also allow oxygen and nitrogen to react and form nitric oxide and nitrogen dioxide, which are responsible for photochemical smog and acid rain. Catalytic converters reduce such emissions by adsorbing CO and NO onto catalytic surface, where the gases undergo a redox reaction. Carbon dioxide and nitrogen are desorbed from the surface and emitted as relatively harmless gases:
- 2CO + 2NO → 2CO2 + N2
Homogeneous catalysts[]
In homogeneous catalysis the catalyst is a molecule which facilitates the reaction. The reactant(s) coordinate to the catalyst (or vice versa), are transformed to product(s), which are then released from the catalyst.
Examples of homogeneous catalysts are H+(aq) which acts as a catalyst in esterification, and chlorine free radicals in the break down of ozone. Chlorine free radicals are formed by the action of ultraviolet radiation on chlorofluorocarbons (CFCs). They react with ozone forming oxygen molecules and regenerating chlorine free radicals:
- Cl + O3 → ClO + O2
- ClO + O → Cl + O2
Biocatalysts[]
In nature enzymes are catalysts in the metabolic pathway. In biochemistry catalysis is also observed with abzymes, ribozymes and deoxyribozymes. In biocatalysis enzymes are used as catalyst in organic chemistry.
Poisoning of a Catalyst[]
- Main article: catalyst poisoning
A catalyst can be poisoned if another compound reacts with it and bonds chemically (similar to an inhibitor) but does not release, or chemically alters the catalyst. This effectively destroys the usefulness of the catalyst, as it cannot participate in the reaction with which it was supposed to catalyse.
Commonly used catalysts[]
Estimates are that 60% of all commercially produced chemical products involve catalysts at some stage in the process of their manufacture.[1]
Some of the most famous catalysts ever developed are:
- Catalytic converters made from platinum and rhodium break down some of the more harmful byproducts of automobile exhaust.
- the Haber process for the synthesis of ammonia from nitrogen and hydrogen, where ordinary iron is used as a catalyst.
Some examples of (famous) catalysts that perform specific transformations on functional groups:
- Transformations of olefinic groups:
- the Ziegler-Natta catalyst used to mass produce polyethylene and polypropylene.
- the Grubbs' catalyst for olefin metathesis.
These given examples show that different catalysts perform other transformations on the same functional groups, where the reaction would not run, run very slow, or would not run in a specific manner without the presence of the catalyst
The most effective catalysts are usually transition metals or transition metal complexes.
See also[]
- Catalysis
- Catalytic converter
- Coordination catalysts
- Enzymes and Ribozymes - biocatalysts
- Nanomaterial based catalysts
References[]
- ↑ "Recognizing the Best in Innovation: Breakthrough Catalyst". R&D Magazine, September 2005, pg 20.
bs:Katalizator ca:Catalitzador cs:Katalyzátor da:Katalysator de:Katalysator et:Katalüsaator es:Catalizador fr:Catalyseur ko:촉매 io:Katalizo id:Katalis he:זרז lt:Katalizatorius mk:Катализатор nl:Katalysator no:Katalysator nn:Katalysator pt:Catalisador ru:Катализатор simple:Catalyst sk:Katalyzátor (chémia) sr:Катализатор su:Katalis fi:Katalyytti sv:Katalysator (kemi) ta:வினைவேக மாற்றி uk:Каталізатор zh:催化剂
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |