Assessment |
Biopsychology |
Comparative |
Cognitive |
Developmental |
Language |
Individual differences |
Personality |
Philosophy |
Social |
Methods |
Statistics |
Clinical |
Educational |
Industrial |
Professional items |
World psychology |
Biological: Behavioural genetics · Evolutionary psychology · Neuroanatomy · Neurochemistry · Neuroendocrinology · Neuroscience · Psychoneuroimmunology · Physiological Psychology · Psychopharmacology (Index, Outline)
| Carbon monoxide | |
|---|---|
 
| |
| General | |
| Systematic name | Carbon monoxide |
| Other names | Carbonic oxide, Coal gas |
| Molecular formula | CO |
| Molar mass | 28.0101 g/mol |
| Appearance | Colorless, odorless gas |
| CAS number | [630-08-0] |
| SMILES | C#O |
| Properties | |
| Density and phase | 0.789 g/cm³, liquid 1.250 g/L at 0°C, 1 atm. 1.145 g/L at 25°C, 1 atm. (lighter than air) |
| Solubility in water | 0.0026 g/100 mL (20 °C) |
| in ethanol in methanol |
Soluble |
| Melting point | -205 °C (68 K) |
| Autoignition temperature | 609 °C |
| Boiling point | -192 °C (81 K) |
| Structure | |
| Molecular shape | Linear |
| Dipole moment | 0.112 D (3.74×10−31 C·m) |
| Hazards | |
| MSDS | External MSDS |
| EU classification | Highly flammable (F+) Repr. Cat. 1 Toxic (T) |
| NFPA 704 |
|
| R-phrases | R12
, R23
, R33
, R48
, |
| S-phrases | S9
, S16
, S33
, S45
, |
| Flash point | Flammable gas |
| RTECS number | FG3500000 |
| Supplementary data page | |
| Structure & properties | n, εr, etc. |
| Thermodynamic data | Phase behaviour Solid, liquid, gas |
| Spectral data | IR = 2143 cm-1 |
| Related compounds | |
| Related oxides | carbon dioxide carbon suboxide dicarbon monoxide carbon trioxide |
| Except where noted otherwise, data are given for materials in their standard state (at 25°C, 100 kPa) Infobox disclaimer and references | |
Carbon monoxide, with the chemical formula CO, is a colourless, odourless, and tasteless gas. It is the product of the incomplete combustion of carbon-containing compounds, notably in internal-combustion engines. It has significant fuel value, burning in air with a characteristic blue flame, producing carbon dioxide. Despite its serious toxicity, CO is extremely valuable and underpins much modern technology, being a precursor to myriad useful — even life-saving — products. It has one carbon molecule and one oxygen molecule.
Role as a neurotransmitter[]
Production[]
Carbon monoxide is so fundamentally important that many methods have been developed for its production.[1]
Producer gas is formed by combustion of carbon in oxygen at high temperatures when there is an excess of carbon. In an oven, air is passed through a bed of coke. The initially produced CO2 equilibrates with the remaining hot carbon to give CO. The reaction of CO2 with carbon to give CO is described as the Boudouard equilibrium. Above 800 °C, CO is the predominant product:
- O2 + 2 C → 2 CO ΔH = -221 kJ/mol
Synthesis gas or Water gas is produced via the endothermic reaction of steam and carbon:
- H2O + C → H2 + CO ΔH = 131 kJ/mol
CO also is a byproduct of the reduction of metal oxide ores with carbon, shown in a simplified form as follows:
- MO + C → M + CO ΔH = 131 kJ/mol
Since CO is a gas, the reduction process can be driven by heating, exploiting the positive (favorable) entropy of reaction. The Ellingham diagram shows that CO formation is favored over CO2 in high temperatures.
CO is the anhydride of formic acid. As such it is conveniently produced by the dehydration of formic acid, for example with sulfuric acid. Another laboratory preparation for carbon monoxide entails heating an intimate mixture of powdered zinc metal and calcium carbonate.
- Zn + CaCO3 → ZnO + CaO + CO
Structure[]
The CO molecule is characterized by a bond length of 0.1128 nm[2]. This distance is consistent with a partial triple bond. The molecule has a small dipole moment and can be represented by three resonance structures:
The leftmost resonance form is the most important.[3] Although one might expect a large dipole moment due to the electronegativity differences between oxygen and carbon, the formal negative charge on the carbon cancels this difference, resulting in a small dipole moment.
Nitrogen is isoelectronic to carbon monoxide, which means that these molecules have the same number of electrons and similar bonding. The physical properties of N2 and CO are similar, although CO is more reactive because it is polar.
Principal chemical reactions[]
Industrial uses[]
Carbon monoxide is a major industrial gas that has many applications in bulk chemicals manufacturing.[4]
High volume aldehydes are produced by the hydroformylation reaction of alkenes, CO, and H2. In one of many applications of this technology, hydroformylation is coupled to the Shell Higher Olefin Process to give precursors to detergents.
Methanol is produced by the hydrogenation of CO. In a related reaction, the hydrogenation of CO is coupled to C-C bond formation, as in the Fischer-Tropsch process where CO is hydrogenated to liquid hydrocarbon fuels. This technology allows coal to be converted to petrol.
In the Monsanto process, carbon monoxide and methanol react in the presence of a homogeneous rhodium catalyst and HI to give acetic acid. This process is responsible for most of the industrial production of acetic acid.
Coordination chemistry[]
- Main article: metal carbonyl
Most metals form coordination complexes containing covalently attached carbon monoxide. In nickel carbonyl, Ni(CO)4 forms by the direct combination of carbon monoxide and nickel metal at room temperature. For this reason, nickel in any tubing or part must not come into prolonged contact with carbon monoxide (corrosion). Nickel carbonyl decomposes readily back to Ni and CO upon contact with hot surfaces, and this method was once used for the industrial purification of nickel in the Mond process.[5]
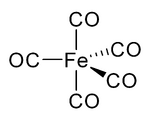
In nickel carbonyl and other carbonyls, the electron pair on the carbon interacts with the metal; the carbon monoxide donates the electron pair to the metal. In these situations, carbon monoxide is called the carbonyl ligand. One of the most important metal carbonyls is Fe(CO)5:
Many metal-CO complexes are prepared by decarbonylation of organic solvents, not from CO. For instance, iridium trichloride and triphenyl phosphine react in boiling methoxyethanol or DMF) to afford IrCl(CO)(PPh3)2.
Organic and main group chemistry[]
In the presence of strong acids and water, carbon monoxide reacts with olefins to form carboxylic acids in a process known as the Koch-Haaf reaction.[6] In the Gattermann-Koch reaction, arenes are converted to benzaldehyde derivatives in the presence of AlCl3 and HCl.[7] Organolithium compounds, e.g. butyl lithium react with CO, but this reaction enjoys little use.
Although CO reacts with carbocations and carbanions, it is relatively unreactive toward organic compounds without the intervention of metal catalysts.[8]
With main group reagents, CO undergoes several noteworthy reactions. Chlorination of CO is the industrial route to the important compound phosgene. With borane CO forms an adduct, H3BCO, which is isoelectronic with the acylium cation [H3CCO]+. CO reacts with sodium to give products resulting from C-C coupling such as Na2C2O2 (sodium acetylenediolate) and Na2C4O4 (sodium squarate).
Carbon monoxide in the atmosphere[]
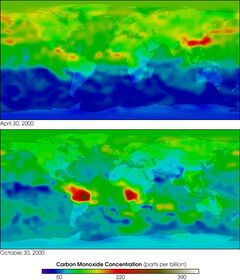
MOPITT 2000 global carbon monoxide
Carbon monoxide, though thought of as a pollutant today, has always been present in the atmosphere, chiefly as a product of volcanic activity. It occurs dissolved in molten volcanic rock at high pressures in the earth's mantle. Carbon monoxide contents of volcanic gases vary from less than 0.01% to as much as 2% depending on the volcano. It also occurs naturally in bushfires. Because natural sources of carbon monoxide are so variable from year to year, it is extremely difficult to accurately measure natural emissions of the gas.
Carbon monoxide has an indirect radiative forcing effect by elevating concentrations of methane and tropospheric ozone through chemical reactions with other atmospheric constituents (e.g., the hydroxyl radical, OH.) that would otherwise destroy them. Carbon monoxide is created when carbon-containing fuels are burned incompletely. Through natural processes in the atmosphere, it is eventually oxidized to carbon dioxide. Carbon monoxide concentrations are both short-lived in the atmosphere and spatially variable.
Anthropogenic CO from automobile and industrial emissions may contribute to the greenhouse effect and global warming. In urban areas carbon monoxide, along with aldehydes, reacts photochemically to produce peroxy radicals. Peroxy radicals react with nitrogen oxide to increase the ratio of NO2 to NO, which reduces the quantity of NO that is available to react with ozone. Carbon monoxide is also a constituent of tobacco smoke.
Role in physiology and food[]
Carbon monoxide is often used to treat fresh seafood and beef consumed in the US. The CO combines with hemoglobin, giving the bright pink color that consumers associate with freshness. The color persists even upon thawing and holding near room temperature thus preserving the fresh look of the product. This treatment is controversial due to concerns that it is misleading and may leave the product appearing fresh even when pathogenic bacteria are present at harmful levels.
One reaction in the body produces CO. Carbon monoxide is produced naturally as a breakdown of hemoglobin, heme, is a substrate for the enzyme heme oxygenase which produces CO and biliverdin. The biliverdin is converted to bilirubin by biliverdin reductase in macrophages of the reticuloendothelial system. The lipid soluble unconjugated bilirubin is transported in the blood bound to albumin, taken up by the hepatocytes, conjugated with glucuronic acid and transported into the bile canaliculi for excretion from the body. The endogenously produced CO may have important physiological roles in the body (eg as a neurotransmitter).
CO is a nutrient for methanogenic bacteria,[9] a building block for acetylcoenzyme A. This theme is the subject for the emerging field of bioorganometallic chemistry. In bacteria, CO is produced via the reduction of carbon dioxide via the enzyme carbon monoxide dehydrogenase, an Fe-Ni-S-containing protein.[10]
A haeme-based CO-sensor protein, CooA, is known.[11] The scope of its biological role is still unclear, it is apparently part of a signalling pathway in bacteria and archaea, but its occurrence in mammals is not established.
CO is also currently being studied for its anti-inflammatory and graft protection properties in the field of transplant immunology.
History[]
Carbon monoxide was first prepared by the French chemist de Lassone in 1776 by heating zinc oxide with coke. He mistakenly concluded that the gaseous product was hydrogen as it burned with a blue flame. The gas was identified as a compound containing carbon and oxygen by the English chemist William Cruikshank in the year 1800.
The toxic properties of CO were first thoroughly investigated by the French physiologist Claude Bernard around 1846. He poisoned dogs with the gas, and noticed that their blood was more rutilant in all the vessels. 'Rutilant' is a French word, but also has an entry in English dictionaries, meaning ruddy, shimmering, or golden. However, it was translated at the time as crimson, scarlet, and now is famously known as 'cherry pink'.
During World War II, carbon monoxide was used to keep motor vehicles running in parts of the world where gasoline was scarce. External charcoal or wood burners were fitted, and the carbon monoxide produced by gasification was piped to the carburetor. The CO in this case is known as "producer gas". Carbon monoxide was also reportedly used on a small scale during the Holocaust at some Nazi extermination camps.
Toxicity[]
- Main article: Carbon monoxide poisoning
CO is a toxic gas. Carbon monoxide detectors for homes are readily available.
See also[]
- Carbon monoxide (data page)
- Boudouard reaction
- List of deaths from carbon monoxide poisoning
Reference[]
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ O.R. Gilliam C.M. Johnson and W. Gordy, Physical. Review volume 78, page 140ff (1950)
- ↑ G. L. Miessler and D. A. Tarr “Inorganic Chemistry” 3rd Ed, Pearson/Prentice Hall publisher, ISBN 0-13-035471-6.
- ↑ Elschenbroich, C.;Salzer, A. ”Organometallics : A Concise Introduction” (2nd Ed) Wiley-VCH: Weinheim, 2006. ISBN 3-527-28165-7
- ↑ Mond L, Langer K, Quincke F (1890). Action of carbon monoxide on nickel. Journal of the Chemical Society: 749-753. DOI
- ↑ Koch, H.; Haaf, W. "1-Adamantanecarboxylic Acid" Organic Syntheses, Collected Volume 5, p.20 (1973).
- ↑ Coleman, G. H.; Craig, D. "p-Tolualdehyde" Organic Syntheses, Collected Volume 2, p.583 (1943).
- ↑ Chatani, N.; Murai, S. "Carbon Monoxide" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. DOI: 10.1002/047084289.
- ↑ Thauer, R. K. "Biochemistry of methanogenesis: a tribute to Marjory Stephenson" Microbiology (1998), volume 144, 2377–2406.
- ↑ Bioorganometallics: Biomolecules, Labeling, Medicine; Jaouen, G., Ed. Wiley-VCH: Weinheim, 2006.3-527-30990-X.
- ↑ Roberts, G. P.; Youn, H.; Kerby, R. L. "CO-Sensing Mechanisms" Microbiology and Molecular Biology Reviews (2004), volume 68, pages 453-473.
External links[]
- International Chemical Safety Card 0023
- National Pollutant Inventory - Carbon Monoxide
- NIOSH Pocket Guide to Chemical Hazards
- CID 281 from PubChem
- United States Environmental Protection Agency Carbon Monoxide page
- External MSDS data sheet
- Carbon Monoxide Kills Campaign Site
- [USFDA IMPORT BULLETIN 16B-95, May 1999]
- [FDA Agency Response Letter GRAS Notice No. GRN 000083]
- Carbon Monoxide in Fresh Meat site]
ar:أول أكسيد الكربون ca:Monòxid de carboni cs:Oxid uhelnatý da:Kulilte de:Kohlenstoffmonoxid es:Monóxido de carbono eo:Karbona monooksido fr:Monoxyde de carbone gl:Monóxido de carbono ko:일산화 탄소 hr:Ugljikov monoksid id:Karbon monoksida is:Kolmónoxíð he:פחמן חד חמצני la:Monoxidum Carbonis lv:Oglekļa monoksīds lt:Anglies monoksidas nl:Koolmonoxide no:Karbonmonoksid nn:Karbonmonoksid pt:Monóxido de carbono ru:Монооксид углерода sk:Oxid uhoľnatý sl:Ogljikov oksid fi:Hiilimonoksidi sv:Kolmonoxid vi:Mônôxít cacbon uk:Монооксид вуглецю zh-yue:一氧化碳 zh:一氧化碳
| This page uses Creative Commons Licensed content from Wikipedia (view authors). |